[ CAS No. 5402-55-1 ] 2-Thiopheneethanol
product standard
| Appearance | Clorless to light yellow transparent liquid |
| Identity Test | Retention time to conforms to reference by GC |
| Purity | 99% min.(GC)) |
| 3-thiopnene ethanol | 0.1% max (GC) |
| Individual impurity (unknown) |
0.2% max(GC) |
| Water content | 0.3% max (W/W) |
| Retest period | One year |
Product Details of [ 5402-55-1 ]
| CAS No. : | 5402-55-1 | MDL No. : | MFCD00005462 |
| Formula : | C6H8OS | Boiling Point : | 223°C at 760 mmHg |
| Linear Structure Formula : | - | InChI Key : | N/A |
| M.W : | 128.19 g/mol | Pubchem ID : | 79400 |
| Synonyms : | |||
Calculated chemistry of of [ 5402-55-1 ]
Physicochemical Properties
| Num. heavy atoms : | 8 |
| Num. arom. heavy atoms : | 5 |
| Fraction Csp3 : | 0.33 |
| Num. rotatable bonds : | 2 |
| Num. H-bond acceptors : | 1.0 |
| Num. H-bond donors : | 1.0 |
| Molar Refractivity : | 35.25 |
| TPSA : | 48.47 Ų |
Pharmacokinetics
| GI absorption : | High |
| BBB permeant : | Yes |
| P-gp substrate : | No |
| CYP1A2 inhibitor : | No |
| CYP2C19 inhibitor : | No |
| CYP2C9 inhibitor : | No |
| CYP2D6 inhibitor : | No |
| CYP3A4 inhibitor : | No |
| Log Kp (skin permeation) : | -6.18 cm/s |
Lipophilicity
| Log Po/w (iLOGP) : | 1.75 |
| Log Po/w (XLOGP3) : | 1.27 |
| Log Po/w (WLOGP) : | 1.28 |
| Log Po/w (MLOGP) : | 0.84 |
| Log Po/w (SILICOS-IT) : | 2.67 |
| Consensus Log Po/w : | 1.56 |
Druglikeness
| Lipinski : | 0.0 |
| Ghose : | None |
| Veber : | 0.0 |
| Egan : | 0.0 |
| Muegge : | 1.0 |
| Bioavailability Score : | 0.55 |
Water Solubility
| Log S (ESOL) : | -1.77 |
| Solubility : | 2.2 mg/ml ; 0.0172 mol/l |
| Class : | Very soluble |
| Log S (Ali) : | -1.89 |
| Solubility : | 1.66 mg/ml ; 0.013 mol/l |
| Class : | Very soluble |
| Log S (SILICOS-IT) : | -1.86 |
| Solubility : | 1.77 mg/ml ; 0.0138 mol/l |
| Class : | Soluble |
Medicinal Chemistry
| PAINS : | 0.0 alert |
| Brenk : | 0.0 alert |
| Leadlikeness : | 1.0 |
| Synthetic accessibility : | 1.82 |
Safety of [ 5402-55-1 ]
| Signal Word: | Warning | Class: | N/A |
| Precautionary Statements: | P261-P305+P351+P338 | UN#: | N/A |
| Hazard Statements: | H302-H315-H319-H335 | Packing Group: | N/A |
| GHS Pictogram: |  |
||
Precautionary Statements-General
| Code | Phrase |
| P101 | If medical advice is needed,have product container or label at hand. |
| P102 | Keep out of reach of children. |
| P103 | Read label before use |
Prevention
| Code | Phrase |
| P201 | Obtain special instructions before use. |
| P202 | Do not handle until all safety precautions have been read and understood. |
| P210 | Keep away from heat/sparks/open flames/hot surfaces. - No smoking. |
| P211 | Do not spray on an open flame or other ignition source. |
| P220 | Keep/Store away from clothing/combustible materials. |
| P221 | Take any precaution to avoid mixing with combustibles |
| P222 | Do not allow contact with air. |
| P223 | Keep away from any possible contact with water, because of violent reaction and possible flash fire. |
| P230 | Keep wetted |
| P231 | Handle under inert gas. |
| P232 | Protect from moisture. |
| P233 | Keep container tightly closed. |
| P234 | Keep only in original container. |
| P235 | Keep cool |
| P240 | Ground/bond container and receiving equipment. |
| P241 | Use explosion-proof electrical/ventilating/lighting/equipment. |
| P242 | Use only non-sparking tools. |
| P243 | Take precautionary measures against static discharge. |
| P244 | Keep reduction valves free from grease and oil. |
| P250 | Do not subject to grinding/shock/friction. |
| P251 | Pressurized container: Do not pierce or burn, even after use. |
| P260 | Do not breathe dust/fume/gas/mist/vapours/spray. |
| P261 | Avoid breathing dust/fume/gas/mist/vapours/spray. |
| P262 | Do not get in eyes, on skin, or on clothing. |
| P263 | Avoid contact during pregnancy/while nursing. |
| P264 | Wash hands thoroughly after handling. |
| P265 | Wash skin thouroughly after handling. |
| P270 | Do not eat, drink or smoke when using this product. |
| P271 | Use only outdoors or in a well-ventilated area. |
| P272 | Contaminated work clothing should not be allowed out of the workplace. |
| P273 | Avoid release to the environment. |
| P280 | Wear protective gloves/protective clothing/eye protection/face protection. |
| P281 | Use personal protective equipment as required. |
| P282 | Wear cold insulating gloves/face shield/eye protection. |
| P283 | Wear fire/flame resistant/retardant clothing. |
| P284 | Wear respiratory protection. |
| P285 | In case of inadequate ventilation wear respiratory protection. |
| P231 + P232 | Handle under inert gas. Protect from moisture. |
| P235 + P410 | Keep cool. Protect from sunlight. |
Response
| Code | Phrase |
| P301 | IF SWALLOWED: |
| P304 | IF INHALED: |
| P305 | IF IN EYES: |
| P306 | IF ON CLOTHING: |
| P307 | IF exposed: |
| P308 | IF exposed or concerned: |
| P309 | IF exposed or if you feel unwell: |
| P310 | Immediately call a POISON CENTER or doctor/physician. |
| P311 | Call a POISON CENTER or doctor/physician. |
| P312 | Call a POISON CENTER or doctor/physician if you feel unwell. |
| P313 | Get medical advice/attention. |
| P314 | Get medical advice/attention if you feel unwell. |
| P315 | Get immediate medical advice/attention. |
| P320 | |
| P302 + P352 | IF ON SKIN: wash with plenty of soap and water. |
| P321 | |
| P322 | |
| P330 | Rinse mouth. |
| P331 | Do NOT induce vomiting. |
| P332 | IF SKIN irritation occurs: |
| P333 | If skin irritation or rash occurs: |
| P334 | Immerse in cool water/wrap n wet bandages. |
| P335 | Brush off loose particles from skin. |
| P336 | Thaw frosted parts with lukewarm water. Do not rub affected area. |
| P337 | If eye irritation persists: |
| P338 | Remove contact lenses, if present and easy to do. Continue rinsing. |
| P340 | Remove victim to fresh air and keep at rest in a position comfortable for breathing. |
| P341 | If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. |
| P342 | If experiencing respiratory symptoms: |
| P350 | Gently wash with plenty of soap and water. |
| P351 | Rinse cautiously with water for several minutes. |
| P352 | Wash with plenty of soap and water. |
| P353 | Rinse skin with water/shower. |
| P360 | Rinse immediately contaminated clothing and skin with plenty of water before removing clothes. |
| P361 | Remove/Take off immediately all contaminated clothing. |
| P362 | Take off contaminated clothing and wash before reuse. |
| P363 | Wash contaminated clothing before reuse. |
| P370 | In case of fire: |
| P371 | In case of major fire and large quantities: |
| P372 | Explosion risk in case of fire. |
| P373 | DO NOT fight fire when fire reaches explosives. |
| P374 | Fight fire with normal precautions from a reasonable distance. |
| P376 | Stop leak if safe to do so. Oxidising gases (section 2.4) 1 |
| P377 | Leaking gas fire: Do not extinguish, unless leak can be stopped safely. |
| P378 | |
| P380 | Evacuate area. |
| P381 | Eliminate all ignition sources if safe to do so. |
| P390 | Absorb spillage to prevent material damage. |
| P391 | Collect spillage. Hazardous to the aquatic environment |
| P301 + P310 | IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
| P301 + P312 | IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
| P301 + P330 + P331 | IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. |
| P302 + P334 | IF ON SKIN: Immerse in cool water/wrap in wet bandages. |
| P302 + P350 | IF ON SKIN: Gently wash with plenty of soap and water. |
| P303 + P361 + P353 | IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
| P304 + P312 | IF INHALED: Call a POISON CENTER or doctor/physician if you feel unwell. |
| P304 + P340 | IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
| P304 + P341 | IF INHALED: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. |
| P305 + P351 + P338 | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| P306 + P360 | IF ON CLOTHING: Rinse Immediately contaminated CLOTHING and SKIN with plenty of water before removing clothes. |
| P307 + P311 | IF exposed: call a POISON CENTER or doctor/physician. |
| P308 + P313 | IF exposed or concerned: Get medical advice/attention. |
| P309 + P311 | IF exposed or if you feel unwell: call a POISON CENTER or doctor/physician. |
| P332 + P313 | IF SKIN irritation occurs: Get medical advice/attention. |
| P333 + P313 | IF SKIN irritation or rash occurs: Get medical advice/attention. |
| P335 + P334 | Brush off loose particles from skin. Immerse in cool water/wrap in wet bandages. |
| P337 + P313 | IF eye irritation persists: Get medical advice/attention. |
| P342 + P311 | IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
| P370 + P376 | In case of fire: Stop leak if safe to Do so. |
| P370 + P378 | In case of fire: |
| P370 + P380 | In case of fire: Evacuate area. |
| P370 + P380 + P375 | In case of fire: Evacuate area. Fight fire remotely due to the risk of explosion. |
| P371 + P380 + P375 | In case of major fire and large quantities: Evacuate area. Fight fire remotely due to the risk of explosion. |
Storage
| Code | Phrase |
| P401 | |
| P402 | Store in a dry place. |
| P403 | Store in a well-ventilated place. |
| P404 | Store in a closed container. |
| P405 | Store locked up. |
| P406 | Store in corrosive resistant/ container with a resistant inner liner. |
| P407 | Maintain air gap between stacks/pallets. |
| P410 | Protect from sunlight. |
| P411 | |
| P412 | Do not expose to temperatures exceeding 50 oC/ 122 oF. |
| P413 | |
| P420 | Store away from other materials. |
| P422 | |
| P402 + P404 | Store in a dry place. Store in a closed container. |
| P403 + P233 | Store in a well-ventilated place. Keep container tightly closed. |
| P403 + P235 | Store in a well-ventilated place. Keep cool. |
| P410 + P403 | Protect from sunlight. Store in a well-ventilated place. |
| P410 + P412 | Protect from sunlight. Do not expose to temperatures exceeding 50 oC/122oF. |
| P411 + P235 | Keep cool. |
Disposal
| Code | Phrase |
| P501 | Dispose of contents/container to ... |
| P502 | Refer to manufacturer/supplier for information on recovery/recycling |
Physical hazards
| Code | Phrase |
| H200 | Unstable explosive |
| H201 | Explosive; mass explosion hazard |
| H202 | Explosive; severe projection hazard |
| H203 | Explosive; fire, blast or projection hazard |
| H204 | Fire or projection hazard |
| H205 | May mass explode in fire |
| H220 | Extremely flammable gas |
| H221 | Flammable gas |
| H222 | Extremely flammable aerosol |
| H223 | Flammable aerosol |
| H224 | Extremely flammable liquid and vapour |
| H225 | Highly flammable liquid and vapour |
| H226 | Flammable liquid and vapour |
| H227 | Combustible liquid |
| H228 | Flammable solid |
| H229 | Pressurized container: may burst if heated |
| H230 | May react explosively even in the absence of air |
| H231 | May react explosively even in the absence of air at elevated pressure and/or temperature |
| H240 | Heating may cause an explosion |
| H241 | Heating may cause a fire or explosion |
| H242 | Heating may cause a fire |
| H250 | Catches fire spontaneously if exposed to air |
| H251 | Self-heating; may catch fire |
| H252 | Self-heating in large quantities; may catch fire |
| H260 | In contact with water releases flammable gases which may ignite spontaneously |
| H261 | In contact with water releases flammable gas |
| H270 | May cause or intensify fire; oxidizer |
| H271 | May cause fire or explosion; strong oxidizer |
| H272 | May intensify fire; oxidizer |
| H280 | Contains gas under pressure; may explode if heated |
| H281 | Contains refrigerated gas; may cause cryogenic burns or injury |
| H290 | May be corrosive to metals |
Health hazards
| Code | Phrase |
| H300 | Fatal if swallowed |
| H301 | Toxic if swallowed |
| H302 | Harmful if swallowed |
| H303 | May be harmful if swallowed |
| H304 | May be fatal if swallowed and enters airways |
| H305 | May be harmful if swallowed and enters airways |
| H310 | Fatal in contact with skin |
| H311 | Toxic in contact with skin |
| H312 | Harmful in contact with skin |
| H313 | May be harmful in contact with skin |
| H314 | Causes severe skin burns and eye damage |
| H315 | Causes skin irritation |
| H316 | Causes mild skin irritation |
| H317 | May cause an allergic skin reaction |
| H318 | Causes serious eye damage |
| H319 | Causes serious eye irritation |
| H320 | Causes eye irritation |
| H330 | Fatal if inhaled |
| H331 | Toxic if inhaled |
| H332 | Harmful if inhaled |
| H333 | May be harmful if inhaled |
| H334 | May cause allergy or asthma symptoms or breathing difficulties if inhaled |
| H335 | May cause respiratory irritation |
| H336 | May cause drowsiness or dizziness |
| H340 | May cause genetic defects |
| H341 | Suspected of causing genetic defects |
| H350 | May cause cancer |
| H351 | Suspected of causing cancer |
| H360 | May damage fertility or the unborn child |
| H361 | Suspected of damaging fertility or the unborn child |
| H361d | Suspected of damaging the unborn child |
| H362 | May cause harm to breast-fed children |
| H370 | Causes damage to organs |
| H371 | May cause damage to organs |
| H372 | Causes damage to organs through prolonged or repeated exposure |
| H373 | May cause damage to organs through prolonged or repeated exposure |
Environmental hazards
| Code | Phrase |
| H400 | Very toxic to aquatic life |
| H401 | Toxic to aquatic life |
| H402 | Harmful to aquatic life |
| H410 | Very toxic to aquatic life with long-lasting effects |
| H411 | Toxic to aquatic life with long-lasting effects |
| H412 | Harmful to aquatic life with long-lasting effects |
| H413 | May cause long-lasting harmful effects to aquatic life |
| H420 | Harms public health and the environment by destroying ozone in the upper atmosphere |
Application In Synthesis of [ 5402-55-1 ]
Upstream synthesis route of [ 5402-55-1 ]
Downstream synthetic route of [ 5402-55-1 ]
Upstream synthesis route of [ 5402-55-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 72% | With strain of the zygomycete fungus S. racemosum MUT 770 In dimethyl sulfoxide for 72 h; Enzymatic reaction | 2.3. Biotransformation experimentsFungal strains were pre-grown in Petri dishes containing maltextract solid medium (MEA: 20 g L−1 glucose, 20 g L−1 malt extract,20 g L−1 agar, 2 g L−1 peptone) from which the inoculum for liquidcultures was set up. The fungus was inoculated as conidia suspen-sion (1 106 conidia/mL) in 50 mL asks containing 40 mL of maltextract liquid medium. Flasks were incubated at 25 C and weremaintained under agitation (110 rpm).After 2 days of pre-growth, a 500 mM solution of the substratein DMSO was added, to a starting substrate concentration (c0) of1–5 mM. For each substrate, three biological replicates were run.The experiment was run for 3 days after the addition of the sub-strates, during which time 1 mL samples were taken, at speciedintervals (usually 24, 48, and 72 h). Each sample was extractedwith EtOAc (500 L), the organic phase was dried over anhydrousNa2SO4 and analysed by means of GC/MS. In some cases (see Section2.4) the isolation of the reduced product has been carried out.For each set of biotransformations, one ask was used to mea-sure the initial biomass and pH before the addition of the substrate.These parameters were also evaluated at the end of the experimentfor all the asks. The liquid media was separated from the biomassby ltration and was used for pH measurement while the myceliawere dried at 60 C for 24 h to measure the biomass dry weight. 2-(Thiophen-2-yl)ethanol: from 2-(thiophen-2-yl)acetic acid(3.7 mg, 72percent) and from methyl 2-(thiophen-2-yl)acetate (24.6 mg,96percent). 1H NMR (400 MHz, CDCl3, TMS): = 7.20 (m, 1H, heteroaro-matic hydrogen), 6.99 (m, 1H, heteroaromatic hydrogen), 6.90 (m,1H, heteroaromatic hydrogen), 3.85 (t, 2H, J = 6.2 Hz, CH2OH), 3.02(t, 2H, J = 6.2 Hz, CH2CH2OH). 13C NMR (100 MHz, CDCl3, TMS): = 140.5, 127.0, 125.8, 124.0, 63.4, 33.3. GC/MS: tR = 9.47 min, m/z128 (M+, 30), 110 (5), 97 (100) |
| 66.5% | Stage #1: With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; Stage #2: With water; sodium chloride In tetrahydrofuran at 0℃; |
Step 9 2-(Thiophen-2-yl)ethanol: At about 0° C., a solution of thiophen-2-yl-acetic acid (1.0 g; 7.03 mmol) in tetrahydrofuran (10 mL) was added dropwise to a suspension of lithium aluminum hydride (0.534 g; 14.05 mmol) in dry tetrahydrofuran (10 mL). The mixture was stirred at ambient temperature for about 4 hours, and then cooled to about 0° C. After adding a cold saturated sodium chloride solution (1 mL), the mixture was filtered, and the inorganic salts were washed with tetrahydrofuran and ethyl acetate. The filtrate and washings were combined and concentrated in vacuo to give the title compound as brown oil (0.600 g; 66.5percent). 1H NMR (400 MHz, CDCl3) δ 1.60 (br, exchangeable with D2O, 1H), 3.08 (t, J=6.2 Hz, 2H), 3.85 (t, J=6.2 Hz, 2H), 6.87-6.88 (m, 1H), 6.95-6.97 (m, 1H), 7.16-7.25 (m, 1H). IR (film) υ 3345, 3105, 2211, 2126, 2090, 1792, 1433, 1138, 972, 737, 699 cm-1 MS: 129 (M+1). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | With strain of the zygomycete fungus S. racemosum MUT 770 In dimethyl sulfoxide for 72 h; Enzymatic reaction | 2.3. Biotransformation experimentsFungal strains were pre-grown in Petri dishes containing maltextract solid medium (MEA: 20 g L−1 glucose, 20 g L−1 malt extract,20 g L−1 agar, 2 g L−1 peptone) from which the inoculum for liquidcultures was set up. The fungus was inoculated as conidia suspen-sion (1 106 conidia/mL) in 50 mL asks containing 40 mL of maltextract liquid medium. Flasks were incubated at 25 C and weremaintained under agitation (110 rpm).After 2 days of pre-growth, a 500 mM solution of the substratein DMSO was added, to a starting substrate concentration (c0) of1–5 mM. For each substrate, three biological replicates were run.The experiment was run for 3 days after the addition of the sub-strates, during which time 1 mL samples were taken, at speciedintervals (usually 24, 48, and 72 h). Each sample was extractedwith EtOAc (500 L), the organic phase was dried over anhydrousNa2SO4 and analysed by means of GC/MS. In some cases (see Section2.4) the isolation of the reduced product has been carried out.For each set of biotransformations, one ask was used to mea-sure the initial biomass and pH before the addition of the substrate.These parameters were also evaluated at the end of the experimentfor all the asks. The liquid media was separated from the biomassby ltration and was used for pH measurement while the myceliawere dried at 60 C for 24 h to measure the biomass dry weight. 2-(Thiophen-2-yl)ethanol: from 2-(thiophen-2-yl)acetic acid(3.7 mg, 72percent) and from methyl 2-(thiophen-2-yl)acetate (24.6 mg,96percent). 1H NMR (400 MHz, CDCl3, TMS): = 7.20 (m, 1H, heteroaro-matic hydrogen), 6.99 (m, 1H, heteroaromatic hydrogen), 6.90 (m,1H, heteroaromatic hydrogen), 3.85 (t, 2H, J = 6.2 Hz, CH2OH), 3.02(t, 2H, J = 6.2 Hz, CH2CH2OH). 13C NMR (100 MHz, CDCl3, TMS): = 140.5, 127.0, 125.8, 124.0, 63.4, 33.3. GC/MS: tR = 9.47 min, m/z128 (M+, 30), 110 (5), 97 (100) |
Downstream synthetic route of [ 5402-55-1 ]
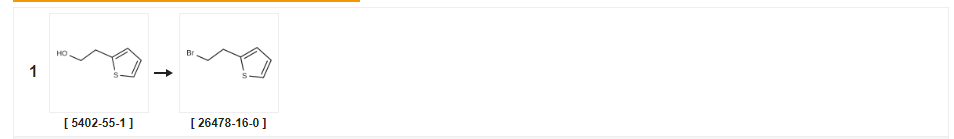
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 94% | With carbon tetrabromide; triphenylphosphine In tetrahydrofuran at 0℃; Schlenk technique; Inert atmosphere; | |
| 69% | With bromine; triphenylphosphine In dichloromethane at 20℃; | |
| 64% | P.31 Synthesis of 2-(2-bromoethyl)thiophene Production Example 31 Synthesis of 2-(2-bromoethyl)thiophene 2-Thienylethanol (0.44 ml) was treated as in the above Production Example 1 to give the title compound (0.490 g) as a colorless oil (yield: 64.0%). 1H-NMR (400 MHz, CDCl3): δ(ppm) 3.38(2H, t, J=7.6Hz), 3.58(2H, t, J=7.6Hz), 6.89(1H, d, J=1.2Hz), 6.96(1H, d, J=4.2Hz), 7.19(1H, dd, J=1.2, 4.2Hz). |
| 28% | With phosphorus tribromide In dichloromethane at 0 - 20℃; for 1h; | |
| 19% | With phosphorus tribromide In tetrachloromethane at 65℃; for 0.333333h; | a Phosphorus tribromide (2.0 mL, 21.1 mmol) was added to a stirred solution of 2-(thiophen-2-yl)ethanol (2.25 g, 17.6 mmol) in carbon tetrachloride (162 mL) then the mixture heated at 65° C. for 20 minutes. The mixture was allowed to cool to ambient temperature then ice added. The organic layer was separated then the aqueous layer extracted with dichloromethane (2×30 mL). The combined organic layers were washed with brine, then dried (NaSO4), filtered and reduced in vacuo. The residue was purified by flash chromatography over silica, eluting with ethyl acetate:hexane mixtures 0:100 to 0.5:95.5 to give 2-(2-bromoethyl)thiophene as a brown oil (650 mg, 19%). |
| With pyridine; chloroform; phosphorus tribromide | ||
| With phosphorus tribromide In diethyl ether at 0℃; for 4h; | 2D Example 2D; 9-(4,5-Dimethyl-thiazol-2-yl)-4-[4-(2-thiophen-2-yl-ethyl)-piperazin-1-yl]-5,6,7, 8- TETRAHYDRO-1, 3, 4B-TRIAZA-FLUORENE To a solution of 2-(2-thienyl) ethanol (1.63g) in dry ether (15mL) at 0°C was added PBr3 (1. 31mL) dropwise. After 4 hours the reaction mixture was diluted with dichloromethane, washed with water, dried (MGS04) and the solvent removed in vacuo to yield a brown oil which was purified using flash chromatography to yield 2- (2-bromo-ethyl)-thiophene. A mixture of 9- (4, 5-DIMETHYL-THIAZOL-2-YL)-4-PIPERAZIN-1-YL-5, 6,7, 8-TETRAHYDRO- 1, 3, 4b-triaza-fluorene (85mg), 2- (2-bromo-ethyl)-thiophene (44mg) and potassium carbonate (38mg) was heated to reflux in acetonitrile (5ML) for 4 hours. The reaction mixture was cooled, extracted into dichloromethane, dried (MGS04) and the solvent removed in vacuo to yield crude product which was purified using flash chromatography to yield the title compound (30mg). |
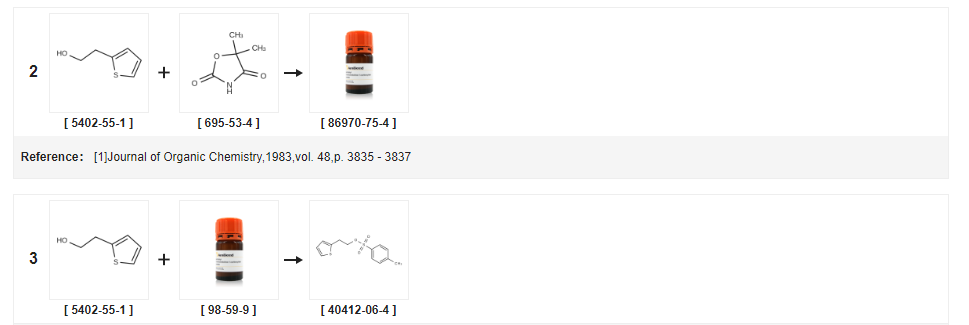
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 98% | With triethylamine; at 35℃;Cooling with ice; | (0.87 mol) of 2-thiopheneethanol and 184 g (0.97 mol) of p-toluenesulfonyl chloride were sequentially added to a 1 L three-necked flask, 98 g (0.97 mol) of triethylamine was dropwise added thereto under ice-water bath, And keep the temperature of the reaction liquid not higher than 20 DEG C, dropping finished, heating to 35 DEG C to continue stirring, respectively 24h, 27h sampling, TLC, until the reaction is complete, stop reaction, filtration, filter cake with appropriate amount of dichloromethane And the methylene chloride layer was dried with anhydrous sodium sulfate for 2 hours. The desiccant was filtered off and the desiccant was washed with a small amount of methylene chloride. The filtrate was decompressed in vacuo and the filtrate was evaporated under reduced pressure. Concentrated to constant weight to be brown oil, weighing 203g, yield 98%, |
| 96.37% | With triethylamine; In toluene; at 5 - 30℃; for 20.8333h;Product distribution / selectivity; | EXAMPLE 3 Preparation of 2-Thienylethyl Para-Toluenesulphonate (Formula VII) Using Toluene 400 liters of toluene and 163.2 kg of para-toluene sulfonyl chloride were charged into a clean and dry reactor followed by cooling to about 5 C. 100 kg of thiophene-2-ethanol was added at about 5 C. over about 20 minutes followed by addition of 130 kg of triethylamine over about 8 hours, 50 minutes. The reaction mixture temperature was raised to about 30 C. followed by stirring for about 12 hours. The reaction mass was filtered through a Nutsche filter and washed with 2*100 liters of toluene. The reaction filtrate was transferred into another reactor followed by washing with 5*200 liters of water. Organic and aqueous layers were separated and the organic layer was distilled completely at about below 70 C. under vacuum to afford 212 kg (yield: 96.37%) of title compound. Purity by GC: 95.59%. |
| 96.5% | With triethylamine; In dichloromethane; at -5 - 20℃; for 2h; | 32.7 g (0.17 mol) of p-toluenesulfonyl chloride,40 ml of dichloromethane into the reaction flask, cooled to -5 C,20 g (0.16 mol)2-thiopheneethanol.28.4 g (0.28 mol) of triethylamine was slowly added dropwise and the temperature of the reaction solution was maintained at about 0 C.Plus Bi, incubated for 2h after the reaction was warmed to room temperature. 2-thiophene ethanol to be consumed until the raw materials, suction filtration,The solid was washed with a small amount of methylene chloride and the filtrate was washed with 50 ml of saturated sodium bicarbonate and dried over anhydrous sodium sulfate. Filtration, concentration of the filtrate, light brown solid precipitated out,Filtered, washed with a small amount of petroleum ether to white,That isP-toluenesulfonate-2-thiophene ethyl ester42.5 g, yield 96.5% (HPLC purity 99%) |
| 95.5% | With triethylamine; In dichloromethane; at 7.5 - 22.5℃; for 5h;Product distribution / selectivity; | EXAMPLE 10 Preparation of 2-(2-Thiophene)Ethanol Tosylate (Formula VII) Using Dichloromethane 4 liters of dichloromethane was added into a reactor at a temperature of about 30 C., cooled to a temperature of about 7.5 C. to which was then added 1.784 kg of p-toluene sulphonyl chloride followed by 1 kg of thiophene-2-ethanol. 1.302 kg of triethylamine was added to the above reaction mass at a temperature of about 7.5 C. followed by slowly raising the temperature of the reaction mass to 22.5 C. for about 5 hours. The obtained reaction mass was filtered through a pressure Nutsche filter, washed with methylene chloride (2*1 liter) and the mother liquor was collected and transferred into another reactor. The organic layer was washed with water (5*2 liters). The organic layer thus obtained was subjected to distillation at a temperature below 70 C. using hot water circulation. The obtained residue was then cooled to about 30 C. to afford 2.1 kg (yield: 95.5%) of title compound. |
| 93.6% | With triethylamine; In toluene; at 45℃; for 4h; | Example 1 0.2. Conversion of (S)-1 ,2,3,4-tetrahydro-5-hvdroxy-N-propyl- naphthalen-2-ammonium hydrobromide (VIII) into hydrochloride salt of rotigotine; 10.2.1. Preparation of 2-(2-Thienyl)ethyl-4-toluene sulfonate; 4-toluenesulfonyl chloride (162 g), toluene (363.3 g) and 2-(2-Thienyl)ethanol (104 g) are combined. Triethylamine (93 g) is added maintaining the temperature lower than 45 C. After 4hrs, the mixture is washed with aqueous phosphoric acid, aqueous sodium hydroxide and then water. The organic phase is distilled off under vacuum. Isopropanol (314 g) and heptanes (365.9 g) are added. The batch is crystallized by cooling and isolated at -15 C. The crystals are filtered and washed with heptanes (175 mL). The crystals are then dried under vacuum at room temperature until a melting point of > 30 C is obtained.Yield ( 214 g): 93.6 %HPLC analyses confirmed purity >99% and 100 % assay in comparison to a reference standard. |
| 93.6% | With triethylamine; In toluene; at 45℃; for 4h; | 4-toluenesulfonyl chloride (162 g), toluene (363.3 g) and 2-(2-Thienyl)ethanol (104 g) are combined. Triethylamine (93 g) is added maintaining the temperature lower than 45 C. After 4 hrs, the mixture is washed with aqueous phosphoric acid, aqueous sodium hydroxide and then water. The organic phase is distilled off under vacuum. Isopropanol (314 g) and heptanes (365.9 g) are added. The batch is crystallized by cooling and isolated at -15 C. The crystals are filtered and washed with heptanes (175 mL). The crystals are then dried under vacuum at room temperature until a melting point of 30 C. is obtained. [0255] Yield (214 g): 93.6% |
| 90% | With silica gel; In dichloromethane; for 2h;Reflux; | The 12.8g (0.1mol) 2- (2- thienyl) ethanol, 1000mL of dichloromethane, 21.0g (0.12mol) of p-toluenesulfonic acid chloride and 10.0g of silica gel into the reaction flask, the reaction was refluxed for 2h, cooled, filtered to remove silica gel.The reaction mixture was washed successively with distilled water, saturated sodium carbonate solution, brine, then the methylene chloride solvent was removed by distillation under reduced pressure, to give 26.0g of p-toluenesulfonic acid Preparation of 2- (2-thienyl) ethyl ester, 90% yield . |
| In pyridine; water; | (a) 2-(2-Hydroxyethyl)thiophene tosylate Toluene-4-sulphonyl chloride (4.125 g) was added portionwise over 5 mins to an ice-cold solution of 2-(2-hydroxyethyl)thiophene (1.723 g) in anhydrous pyridine (20 ml) and the resulting pale yellow solution was stirred at 0 C. After 3 h the reaction mixture was poured into vigorously stirred water (160 ml) producing a precipitate. After cooling the solid was collected and washed with water to give white crystals of the title tosylate (3.555 g), m.p. 33-34 C., lambdamax (EtOH) 227 nm (E11 612). | |
| With triethylamine; In butanone; at 0 - 30℃; | Example 1 Preparation of 2-(2-Thienyl)-ethyl para-toluenesulfonate A mixture of p-toluenesulfonyl chloride (328 gm) and 2-(2-thienyl)-ethanol (200 gm) in methyl ethyl ketone (1000 ml) was cooled to 0C. This was followed by drop wise addition of triethyl amine (283.1 ml) at 0-5C over a period of 1 to 2 hours, and the reaction mass was stirred for 12 to 15 hours at 25-3O0C. The resulting mass was filtered and washed with methyl ethyl ketone (500 ml). The resulting organic layer was washed with water (500 ml) followed by washings with saturated sodium bicarbonate solution (500 ml) and brine solution (500 ml). The resulting organic layer was distilled under vacuum at below 50C to give 2-(2- thienyl)-ethyl para-toluenesulfonate as an oily mass (Weight of the oil: 505 gm; Purity by HPLC: 97%). | |
| With triethylamine; In butanone; at 0 - 30℃; | Example 1 Preparation of 2-(2-Thienyl)-ethyl para-toluenesulfonate A mixture of p-toluenesulfonyl chloride (328 gm) and 2-(2-thienyl)-ethanol (200 gm) in methyl ethyl ketone (1000 ml) was cooled to 0 C. This was followed by drop wise addition of triethyl amine (283.1 ml) at 0-5 C. over a period of 1 to 2 hours, and the reaction mass was stirred for 12 to 15 hours at 25-30 C. The resulting mass was filtered and washed with methyl ethyl ketone (500 ml). The resulting organic layer was washed with water (500 ml) followed by washings with saturated sodium bicarbonate solution (500 ml) and brine solution (500 ml). The resulting organic layer was distilled under vacuum at below 50 C. to give 2-(2-thienyl)-ethyl para-toluenesulfonate as an oily mass (Weight of the oil: 505 gm; Purity by HPLC: 97%). | |
| With potassium carbonate; In toluene; at 0 - 20℃; for 3.5h; | 500 ml of toluene was added to a 1000 ml reaction flask,50gThiophene ethanolAnd 80 g of p-toluenesulfonyl chloride,Turn on agitation,Controlled temperature was added dropwise at 0 C64 g of potassium carbonate,The addition was continued for about 30 min, Dropping finished warming to 20 C reaction for 3 hours,To the reaction solution was added 400 ml of water,Washed twice,The washed toluene layer was directly used for the next reaction. | |
| With N-ethyl-N,N-diisopropylamine; In toluene; at 0 - 20℃; for 3.5h; | 500 ml of toluene was added to a 1000 ml reaction flask, 50 g of thiophene ethanol and 92 g of p-toluenesulfonyl chloride, stirred at 0 C, 62 g of N, N-diisopropylethylamine was dropped for about 30min. When dropping was completed, mixture was heated to 20 C and reacted for 3 hours. To the reaction solution was added 400 ml of water, washed twice, the washed toluene layer was directly used for the next reaction. | |
| With triethylamine; In toluene; at 0 - 20℃; for 3h; | 500 ml of toluene, 50 g of thiophene ethanol and 80 g of p-toluenesulfonyl chloride were placed in a 1000 ml reaction flask,And the temperature was raised to 20 C for 3 hours. 400 ml of water was added to the reaction solution, washed twice, washed with toluene, Layer directly to the next step reaction. | |
| With potassium carbonate; In toluene; at 0 - 20℃; for 3.5h; | 500 ml of toluene, 50 g of thiophene ethanol and 107 g of p-toluenesulfonyl chloride were placed in a 1000 ml reaction flask, agitation was turned on, the temperature was controlled at 0 C, dropwise addition of 64g potassium carbonate, addition lasted about 30min, after which it was warmed to 20 C and reacted for 3 hours, to the reaction solution was added 400 ml of water, washed twice, and the washed toluene layer was directly used for the next reaction. | |
| With triethylamine; In toluene; at 0 - 25℃; for 3.5h; | Example 1The preparation method of ticlopidine hydrochloride as shown in Fig. 2 comprises the following steps:1. p-toluenesulfonyl protection:In a 1000 ml reaction flask, 500 ml of toluene, 50 g of thiophene ethanol and 80 g of p-toluenesulfonyl chloride were charged and stirred to controlAt a temperature of 0 C, 47 g of triethylamine was added dropwise, dropping for about 30 min, and the temperature was raised to 25 C for 3 hours.Add 400ml of water, washed twice, washed with toluene layer directly to the next step reaction.2. Condensation reactionThe reaction solution of toluene in the previous step was added to a 1000 ml reaction flask, followed by the addition of 114 g of o-chlorobenzylamine and heating to 90 C for 3 hours. After the reaction, the mixture was cooled to 25 C and stirred for 1 hour. The filtrate was added with 200 ml of water, and hydrochloric acidAdjust the pH of the system to 8.5, stratify the upper layer of toluene and continue dropping hydrochloric acid to adjust the pH to 5, then cool the system to 2 CThe crystals were mixed for 4 hours and filtered, and the filter cake was dried in vacuo at 50 C to give 96 g of the condensate hydrochloride.3 ? Closed loop reactionTo the 1000 ml reaction flask was added 96 g of the condensate hydrochloride, 400 ml of 1,3-dioxane and 5 ml of hydrochloric acid,To 90 C for 6 hours. After the reaction, the mixture was cooled to 7 C and stirred for 3 hours. The filter cake was washed with a small amount of 1,3-dioxetaneAfter 50 C vacuum drying, 95 g of ticlopidine hydrochloride was obtained4 ? RefinedIn a 1000 ml reaction flask, 95 g of crude ticlopidine hydrochloride and 500 ml of absolute ethanol were added and heated to 72 C with stirring,About 10 minutes after the solid is completely dissolved by adding 2g activated carbon, insulation bleaching 20 minutes after the hot filter, the filtrate gradually coolingThe crystals were crystallized at 4 C for 4 hours and filtered. The filter cake was washed with a small amount of absolute ethanol and dried in vacuo at 50 C to give 91 g of tithiol hydrochloridePrecision products. Total yield 82%, purity 99.9% | |
| With sodium hydroxide; In water; toluene; at 10 - 55℃; for 11h; | In a three-necked flask, 97 g of p-toluenesulfonyl chloride and 180 ml of toluene were placed.Stir and dissolve, filter, keep warm at 15-20 C,60 g of 2-thiopheneethanol and 60 ml of toluene were placed in a three-necked flask.120g 40% aqueous sodium hydroxide, stirring,A solution of 97 g of p-toluenesulfonyl chloride dissolved in 180 ml of toluene was added dropwise at 10-20 C, and the mixture was stirred for 3 h, then heated to 45-55 C for 8 h, and allowed to stand for stratification.The organic phase is depressurized to remove toluene to give 2-(2-thiophene)ethanol p-toluenesulfonate.Reddish brown oil; the molecular formula is as follows: | |
| 235.1 g | With sodium hydroxide; In chloroform; at 0 - 5℃; for 12h; | To the reaction flask, the above step 2-thiophene ethanol: 112 g, chloroform 600 g, PTSC 249.4 g, and the mixture was cooled to 0-5 C.Add 834g of 10% by mass sodium hydroxide solution, control the temperature 0-5 C, keep warm for 12h, add hydrochloric acid to pH=12, layer, the water layer is extracted with 300g chloroform, and the chloroform layer is combined. After washing with 100 g of water, the organic layer was distilled to dryness to give 2-thiopheneethanol p-toluenesulfonate: 235.1 g yield 95.3%, purity 99.5%. |
Write your message here and send it to us

![[ CAS No. 5402-55-1 ] 2-Thiopheneethanol Featured Image](https://www.ohlsenwin.com/uploads/p1.png)
![[ CAS No. 5402-55-1 ] 2-Thiopheneethanol](https://www.ohlsenwin.com/uploads/p1-300x300.png)
![[ CAS No. 5402-55-1 ] 2-Thiopheneethanol](https://www.ohlsenwin.com/uploads/product-2-300x300.png)
![[ CAS No. 5402-55-1 ] 2-Thiopheneethanol](https://www.ohlsenwin.com/uploads/product-1-300x300.png)





![[ CAS No. 141109-19-5 ] (S)-Methyl 2-(2-chlorophenyl)-2-((2-(thiophen-2-yl)ethyl)amino)acetate hydrochloride](https://www.ohlsenwin.com/uploads/Chlorophenylglycine-methyl-ester-31-300x300.png)
![[ CAS No. 40412-06-4 ] 2-(Thiophen-2-yl)ethyl 4-methylbenzenesulfonate](https://www.ohlsenwin.com/uploads/ethanol-Tosylate-4-300x300.png)
![[ CAS No. 141109-14-0 ] (S)-Methyl 2-amino-2-(2-chlorophenyl)acetate](https://www.ohlsenwin.com/uploads/Chlorophenylglycine-methyl-ester-2-300x300.png)